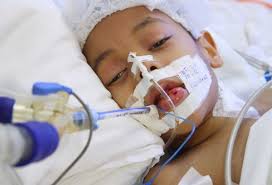
New research suggests that certain small molecules used by cells to control the proteins they make might also help doctors identify adult acute-leukemia patients who are likely to respond poorly to therapy.
Researchers say the findings should improve the understanding of acute myeloid leukemia (AML) and could lead to new therapies for patients with few treatment options.
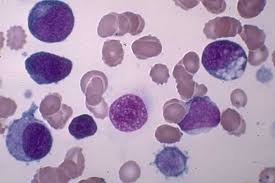
The study examined the levels of molecules called microRNAs in leukemia cells from 122 patients with high- and intermediate-risk AML and in normal blood stem cells from 10 healthy donors.
The findings showed that both the leukemia cells and their normal counterparts had similar kinds of microRNA, but that the two groups differed in the levels of miRNAs present.
The research also identified two microRNAs present at abnormally high levels that were clearly associated with patient survival.
The investigators verified their findings in an additional group of 60 patients using a different technology.
The study, published online Jan.10, 2008 in the journal Blood, was led by researchers with The Ohio State University Comprehensive Cancer Center and the M.D. Anderson Cancer Center.
“If our results are validated by other groups, these two elevated microRNAs can be used to determine which patients require more aggressive treatment,” says first author Dr. Ramiro Garzon, assistant professor of internal medicine and a researcher with The Ohio State University Comprehensive Cancer Center.
“In addition, they may provide new targets for future therapies – knocking out these two microRNAs might benefit patients who have a poor prognosis.”
This possibility is particularly intriguing, he says, because the two microRNAs – called miR-191 and miR-199a – are also associated with cancers of the lung, prostate, colon, stomach and breast. This suggests that they may be part of a common cancer pathway.
Garzon noted that the study also found an association between high levels of a microRNA called miR-155 in AML patients and a gene mutation called FLT3-ITD. High levels of this microRNA have been reported in other cancers and to cause leukemia in mice.
“Clearly, our findings suggest that the quantity of microRNAs present is important in cancer, suggesting that modulating their levels might offer an effective way to treat the disease in these patients,” he says.
For this study, Garzon and his colleagues used blood samples from newly diagnosed AML patients who had either normal-looking chromosomes, a feature that indicates intermediate risk of recurrence, or other chromosome alterations. These included isolated trisomy 8, the t(11q23) translocation and multiple chromosomal abnormalities that signal a high risk of recurrence.
Together, these groups make up the majority of the 13,400 people expected to be diagnosed with AML in 2007. About 9,000 people that year were expected to die of the disease.
“Our efforts now should concentrate on characterizing how these altered microRNAs might promote leukemia and on developing drugs designed to inhibit their action,” Garzon says.
Funding from the National Cancer Institute, The Paul and Mary Haas Chair in Genetics, a Lauri Strauss Discovery Grant, the Kimmel Cancer Research Foundation, the CLL Global Research Foundation, the AIRC and PRRIITT Regione Emilia Romagna GebbaLab, and the Leukemia Clinical Research Foundation supported this research.
Other Ohio State researchers involved in this study were Stefano Volinia, Chang-Gong Liu, Cecilia Fernandez-Cymering, Tiziana Palumbo, Flavia Pichiorri, Muller Fabbri, Hansjuerg Alder, Tatsuya Nakamura, Guido Marcucci, Clara D. Bloomfield and Carlo M. Croce.New research suggests that certain small molecules used by cells to control the proteins they make might also help doctors identify adult acute-leukemia patients who are likely to respond poorly to therapy.
Researchers say the findings should improve the understanding of acute myeloid leukemia (AML) and could lead to new therapies for patients with few treatment options.
The study examined the levels of molecules called microRNAs in leukemia cells from 122 patients with high- and intermediate-risk AML and in normal blood stem cells from 10 healthy donors.
The findings showed that both the leukemia cells and their normal counterparts had similar kinds of microRNA, but that the two groups differed in the levels of miRNAs present.
The research also identified two microRNAs present at abnormally high levels that were clearly associated with patient survival.
The investigators verified their findings in an additional group of 60 patients using a different technology.
The study, published online Jan.10, 2008 in the journal Blood, was led by researchers with The Ohio State University Comprehensive Cancer Center and the M.D. Anderson Cancer Center.
“If our results are validated by other groups, these two elevated microRNAs can be used to determine which patients require more aggressive treatment,” says first author Dr. Ramiro Garzon, assistant professor of internal medicine and a researcher with The Ohio State University Comprehensive Cancer Center.
“In addition, they may provide new targets for future therapies – knocking out these two microRNAs might benefit patients who have a poor prognosis.”
This possibility is particularly intriguing, he says, because the two microRNAs – called miR-191 and miR-199a – are also associated with cancers of the lung, prostate, colon, stomach and breast. This suggests that they may be part of a common cancer pathway.
Garzon noted that the study also found an association between high levels of a microRNA called miR-155 in AML patients and a gene mutation called FLT3-ITD. High levels of this microRNA have been reported in other cancers and to cause leukemia in mice.
“Clearly, our findings suggest that the quantity of microRNAs present is important in cancer, suggesting that modulating their levels might offer an effective way to treat the disease in these patients,” he says.
For this study, Garzon and his colleagues used blood samples from newly diagnosed AML patients who had either normal-looking chromosomes, a feature that indicates intermediate risk of recurrence, or other chromosome alterations. These included isolated trisomy 8, the t(11q23) translocation and multiple chromosomal abnormalities that signal a high risk of recurrence.
Together, these groups make up the majority of the 13,400 people expected to be diagnosed with AML in 2007. About 9,000 people that year were expected to die of the disease.
“Our efforts now should concentrate on characterizing how these altered microRNAs might promote leukemia and on developing drugs designed to inhibit their action,” Garzon says.
Funding from the National Cancer Institute, The Paul and Mary Haas Chair in Genetics, a Lauri Strauss Discovery Grant, the Kimmel Cancer Research Foundation, the CLL Global Research Foundation, the AIRC and PRRIITT Regione Emilia Romagna GebbaLab, and the Leukemia Clinical Research Foundation supported this research.
Other Ohio State researchers involved in this study were Stefano Volinia, Chang-Gong Liu, Cecilia Fernandez-Cymering, Tiziana Palumbo, Flavia Pichiorri, Muller Fabbri, Hansjuerg Alder, Tatsuya Nakamura, Guido Marcucci, Clara D. Bloomfield and Carlo M. Croce.New research suggests that certain small molecules used by cells to control the proteins they make might also help doctors identify adult acute-leukemia patients who are likely to respond poorly to therapy.
Researchers say the findings should improve the understanding of acute myeloid leukemia (AML) and could lead to new therapies for patients with few treatment options.
The study examined the levels of molecules called microRNAs in leukemia cells from 122 patients with high- and intermediate-risk AML and in normal blood stem cells from 10 healthy donors.
The findings showed that both the leukemia cells and their normal counterparts had similar kinds of microRNA, but that the two groups differed in the levels of miRNAs present.
The research also identified two microRNAs present at abnormally high levels that were clearly associated with patient survival.
The investigators verified their findings in an additional group of 60 patients using a different technology.
The study, published online Jan.10, 2008 in the journal Blood, was led by researchers with The Ohio State University Comprehensive Cancer Center and the M.D. Anderson Cancer Center.
“If our results are validated by other groups, these two elevated microRNAs can be used to determine which patients require more aggressive treatment,” says first author Dr. Ramiro Garzon, assistant professor of internal medicine and a researcher with The Ohio State University Comprehensive Cancer Center.
“In addition, they may provide new targets for future therapies – knocking out these two microRNAs might benefit patients who have a poor prognosis.”
This possibility is particularly intriguing, he says, because the two microRNAs – called miR-191 and miR-199a – are also associated with cancers of the lung, prostate, colon, stomach and breast. This suggests that they may be part of a common cancer pathway.
Garzon noted that the study also found an association between high levels of a microRNA called miR-155 in AML patients and a gene mutation called FLT3-ITD. High levels of this microRNA have been reported in other cancers and to cause leukemia in mice.
“Clearly, our findings suggest that the quantity of microRNAs present is important in cancer, suggesting that modulating their levels might offer an effective way to treat the disease in these patients,” he says.
For this study, Garzon and his colleagues used blood samples from newly diagnosed AML patients who had either normal-looking chromosomes, a feature that indicates intermediate risk of recurrence, or other chromosome alterations. These included isolated trisomy 8, the t(11q23) translocation and multiple chromosomal abnormalities that signal a high risk of recurrence.
Together, these groups make up the majority of the 13,400 people expected to be diagnosed with AML in 2007. About 9,000 people that year were expected to die of the disease.
“Our efforts now should concentrate on characterizing how these altered microRNAs might promote leukemia and on developing drugs designed to inhibit their action,” Garzon says.
Funding from the National Cancer Institute, The Paul and Mary Haas Chair in Genetics, a Lauri Strauss Discovery Grant, the Kimmel Cancer Research Foundation, the CLL Global Research Foundation, the AIRC and PRRIITT Regione Emilia Romagna GebbaLab, and the Leukemia Clinical Research Foundation supported this research.
Other Ohio State researchers involved in this study were Stefano Volinia, Chang-Gong Liu, Cecilia Fernandez-Cymering, Tiziana Palumbo, Flavia Pichiorri, Muller Fabbri, Hansjuerg Alder, Tatsuya Nakamura, Guido Marcucci, Clara D. Bloomfield and Carlo M. Croce.New research suggests that certain small molecules used by cells to control the proteins they make might also help doctors identify adult acute-leukemia patients who are likely to respond poorly to therapy.
Researchers say the findings should improve the understanding of acute myeloid leukemia (AML) and could lead to new therapies for patients with few treatment options.
The study examined the levels of molecules called microRNAs in leukemia cells from 122 patients with high- and intermediate-risk AML and in normal blood stem cells from 10 healthy donors.
The findings showed that both the leukemia cells and their normal counterparts had similar kinds of microRNA, but that the two groups differed in the levels of miRNAs present.
The research also identified two microRNAs present at abnormally high levels that were clearly associated with patient survival.
The investigators verified their findings in an additional group of 60 patients using a different technology.
The study, published online Jan.10, 2008 in the journal Blood, was led by researchers with The Ohio State University Comprehensive Cancer Center and the M.D. Anderson Cancer Center.
“If our results are validated by other groups, these two elevated microRNAs can be used to determine which patients require more aggressive treatment,” says first author Dr. Ramiro Garzon, assistant professor of internal medicine and a researcher with The Ohio State University Comprehensive Cancer Center.
“In addition, they may provide new targets for future therapies – knocking out these two microRNAs might benefit patients who have a poor prognosis.”
This possibility is particularly intriguing, he says, because the two microRNAs – called miR-191 and miR-199a – are also associated with cancers of the lung, prostate, colon, stomach and breast. This suggests that they may be part of a common cancer pathway.
Garzon noted that the study also found an association between high levels of a microRNA called miR-155 in AML patients and a gene mutation called FLT3-ITD. High levels of this microRNA have been reported in other cancers and to cause leukemia in mice.
“Clearly, our findings suggest that the quantity of microRNAs present is important in cancer, suggesting that modulating their levels might offer an effective way to treat the disease in these patients,” he says.
For this study, Garzon and his colleagues used blood samples from newly diagnosed AML patients who had either normal-looking chromosomes, a feature that indicates intermediate risk of recurrence, or other chromosome alterations. These included isolated trisomy 8, the t(11q23) translocation and multiple chromosomal abnormalities that signal a high risk of recurrence.
Together, these groups make up the majority of the 13,400 people expected to be diagnosed with AML in 2007. About 9,000 people that year were expected to die of the disease.
“Our efforts now should concentrate on characterizing how these altered microRNAs might promote leukemia and on developing drugs designed to inhibit their action,” Garzon says.
Funding from the National Cancer Institute, The Paul and Mary Haas Chair in Genetics, a Lauri Strauss Discovery Grant, the Kimmel Cancer Research Foundation, the CLL Global Research Foundation, the AIRC and PRRIITT Regione Emilia Romagna GebbaLab, and the Leukemia Clinical Research Foundation supported this research.
Other Ohio State researchers involved in this study were Stefano Volinia, Chang-Gong Liu, Cecilia Fernandez-Cymering, Tiziana Palumbo, Flavia Pichiorri, Muller Fabbri, Hansjuerg Alder, Tatsuya Nakamura, Guido Marcucci, Clara D. Bloomfield and Carlo M. Croce.New research suggests that certain small molecules used by cells to control the proteins they make might also help doctors identify adult acute-leukemia patients who are likely to respond poorly to therapy.
Researchers say the findings should improve the understanding of acute myeloid leukemia (AML) and could lead to new therapies for patients with few treatment options.
The study examined the levels of molecules called microRNAs in leukemia cells from 122 patients with high- and intermediate-risk AML and in normal blood stem cells from 10 healthy donors.
The findings showed that both the leukemia cells and their normal counterparts had similar kinds of microRNA, but that the two groups differed in the levels of miRNAs present.
The research also identified two microRNAs present at abnormally high levels that were clearly associated with patient survival.
The investigators verified their findings in an additional group of 60 patients using a different technology.
The study, published online Jan.10, 2008 in the journal Blood, was led by researchers with The Ohio State University Comprehensive Cancer Center and the M.D. Anderson Cancer Center.
“If our results are validated by other groups, these two elevated microRNAs can be used to determine which patients require more aggressive treatment,” says first author Dr. Ramiro Garzon, assistant professor of internal medicine and a researcher with The Ohio State University Comprehensive Cancer Center.
“In addition, they may provide new targets for future therapies – knocking out these two microRNAs might benefit patients who have a poor prognosis.”
This possibility is particularly intriguing, he says, because the two microRNAs – called miR-191 and miR-199a – are also associated with cancers of the lung, prostate, colon, stomach and breast. This suggests that they may be part of a common cancer pathway.
Garzon noted that the study also found an association between high levels of a microRNA called miR-155 in AML patients and a gene mutation called FLT3-ITD. High levels of this microRNA have been reported in other cancers and to cause leukemia in mice.
“Clearly, our findings suggest that the quantity of microRNAs present is important in cancer, suggesting that modulating their levels might offer an effective way to treat the disease in these patients,” he says.
For this study, Garzon and his colleagues used blood samples from newly diagnosed AML patients who had either normal-looking chromosomes, a feature that indicates intermediate risk of recurrence, or other chromosome alterations. These included isolated trisomy 8, the t(11q23) translocation and multiple chromosomal abnormalities that signal a high risk of recurrence.
Together, these groups make up the majority of the 13,400 people expected to be diagnosed with AML in 2007. About 9,000 people that year were expected to die of the disease.
“Our efforts now should concentrate on characterizing how these altered microRNAs might promote leukemia and on developing drugs designed to inhibit their action,” Garzon says.
Funding from the National Cancer Institute, The Paul and Mary Haas Chair in Genetics, a Lauri Strauss Discovery Grant, the Kimmel Cancer Research Foundation, the CLL Global Research Foundation, the AIRC and PRRIITT Regione Emilia Romagna GebbaLab, and the Leukemia Clinical Research Foundation supported this research.
Other Ohio State researchers involved in this study were Stefano Volinia, Chang-Gong Liu, Cecilia Fernandez-Cymering, Tiziana Palumbo, Flavia Pichiorri, Muller Fabbri, Hansjuerg Alder, Tatsuya Nakamura, Guido Marcucci, Clara D. Bloomfield and Carlo M. Croce.New research suggests that certain small molecules used by cells to control the proteins they make might also help doctors identify adult acute-leukemia patients who are likely to respond poorly to therapy.
Researchers say the findings should improve the understanding of acute myeloid leukemia (AML) and could lead to new therapies for patients with few treatment options.
The study examined the levels of molecules called microRNAs in leukemia cells from 122 patients with high- and intermediate-risk AML and in normal blood stem cells from 10 healthy donors.
The findings showed that both the leukemia cells and their normal counterparts had similar kinds of microRNA, but that the two groups differed in the levels of miRNAs present.
The research also identified two microRNAs present at abnormally high levels that were clearly associated with patient survival.
The investigators verified their findings in an additional group of 60 patients using a different technology.
The study, published online Jan.10, 2008 in the journal Blood, was led by researchers with The Ohio State University Comprehensive Cancer Center and the M.D. Anderson Cancer Center.
“If our results are validated by other groups, these two elevated microRNAs can be used to determine which patients require more aggressive treatment,” says first author Dr. Ramiro Garzon, assistant professor of internal medicine and a researcher with The Ohio State University Comprehensive Cancer Center.
“In addition, they may provide new targets for future therapies – knocking out these two microRNAs might benefit patients who have a poor prognosis.”
This possibility is particularly intriguing, he says, because the two microRNAs – called miR-191 and miR-199a – are also associated with cancers of the lung, prostate, colon, stomach and breast. This suggests that they may be part of a common cancer pathway.
Garzon noted that the study also found an association between high levels of a microRNA called miR-155 in AML patients and a gene mutation called FLT3-ITD. High levels of this microRNA have been reported in other cancers and to cause leukemia in mice.
“Clearly, our findings suggest that the quantity of microRNAs present is important in cancer, suggesting that modulating their levels might offer an effective way to treat the disease in these patients,” he says.
For this study, Garzon and his colleagues used blood samples from newly diagnosed AML patients who had either normal-looking chromosomes, a feature that indicates intermediate risk of recurrence, or other chromosome alterations. These included isolated trisomy 8, the t(11q23) translocation and multiple chromosomal abnormalities that signal a high risk of recurrence.
Together, these groups make up the majority of the 13,400 people expected to be diagnosed with AML in 2007. About 9,000 people that year were expected to die of the disease.
“Our efforts now should concentrate on characterizing how these altered microRNAs might promote leukemia and on developing drugs designed to inhibit their action,” Garzon says.
Funding from the National Cancer Institute, The Paul and Mary Haas Chair in Genetics, a Lauri Strauss Discovery Grant, the Kimmel Cancer Research Foundation, the CLL Global Research Foundation, the AIRC and PRRIITT Regione Emilia Romagna GebbaLab, and the Leukemia Clinical Research Foundation supported this research.
Other Ohio State researchers involved in this study were Stefano Volinia, Chang-Gong Liu, Cecilia Fernandez-Cymering, Tiziana Palumbo, Flavia Pichiorri, Muller Fabbri, Hansjuerg Alder, Tatsuya Nakamura, Guido Marcucci, Clara D. Bloomfield and Carlo M. Croce.New research suggests that certain small molecules used by cells to control the proteins they make might also help doctors identify adult acute-leukemia patients who are likely to respond poorly to therapy.
Researchers say the findings should improve the understanding of acute myeloid leukemia (AML) and could lead to new therapies for patients with few treatment options.
The study examined the levels of molecules called microRNAs in leukemia cells from 122 patients with high- and intermediate-risk AML and in normal blood stem cells from 10 healthy donors.
The findings showed that both the leukemia cells and their normal counterparts had similar kinds of microRNA, but that the two groups differed in the levels of miRNAs present.
The research also identified two microRNAs present at abnormally high levels that were clearly associated with patient survival.
The investigators verified their findings in an additional group of 60 patients using a different technology.
The study, published online Jan.10, 2008 in the journal Blood, was led by researchers with The Ohio State University Comprehensive Cancer Center and the M.D. Anderson Cancer Center.
“If our results are validated by other groups, these two elevated microRNAs can be used to determine which patients require more aggressive treatment,” says first author Dr. Ramiro Garzon, assistant professor of internal medicine and a researcher with The Ohio State University Comprehensive Cancer Center.
“In addition, they may provide new targets for future therapies – knocking out these two microRNAs might benefit patients who have a poor prognosis.”
This possibility is particularly intriguing, he says, because the two microRNAs – called miR-191 and miR-199a – are also associated with cancers of the lung, prostate, colon, stomach and breast. This suggests that they may be part of a common cancer pathway.
Garzon noted that the study also found an association between high levels of a microRNA called miR-155 in AML patients and a gene mutation called FLT3-ITD. High levels of this microRNA have been reported in other cancers and to cause leukemia in mice.
“Clearly, our findings suggest that the quantity of microRNAs present is important in cancer, suggesting that modulating their levels might offer an effective way to treat the disease in these patients,” he says.
For this study, Garzon and his colleagues used blood samples from newly diagnosed AML patients who had either normal-looking chromosomes, a feature that indicates intermediate risk of recurrence, or other chromosome alterations. These included isolated trisomy 8, the t(11q23) translocation and multiple chromosomal abnormalities that signal a high risk of recurrence.
Together, these groups make up the majority of the 13,400 people expected to be diagnosed with AML in 2007. About 9,000 people that year were expected to die of the disease.
“Our efforts now should concentrate on characterizing how these altered microRNAs might promote leukemia and on developing drugs designed to inhibit their action,” Garzon says.
Funding from the National Cancer Institute, The Paul and Mary Haas Chair in Genetics, a Lauri Strauss Discovery Grant, the Kimmel Cancer Research Foundation, the CLL Global Research Foundation, the AIRC and PRRIITT Regione Emilia Romagna GebbaLab, and the Leukemia Clinical Research Foundation supported this research.
Other Ohio State researchers involved in this study were Stefano Volinia, Chang-Gong Liu, Cecilia Fernandez-Cymering, Tiziana Palumbo, Flavia Pichiorri, Muller Fabbri, Hansjuerg Alder, Tatsuya Nakamura, Guido Marcucci, Clara D. Bloomfield and Carlo M. Croce.
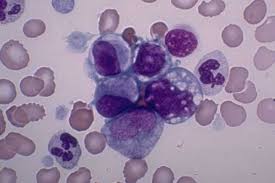




 A new case study by Mayo Clinic researchers provides preliminary evidence to suggest a component of green tea may lead to clinical improvement in patients with chronic lymphocytic leukemia (CLL). Findings are published online in Leukemia Research. In the small case study, the researchers report on four patients who appeared to have an improvement in the clinical state of their disease after starting over-the-counter products containing epigallocatechin gallate (EGCG), an extract of green tea. Three of the four patients met the standard criteria used to define a response treatment for clinical trials. These same investigators had previously shown that EGCG kills leukemia cells from patients with CLL in the test tube by interrupting the communication signals they need to survive. That study was published in Blood in 2004.
A new case study by Mayo Clinic researchers provides preliminary evidence to suggest a component of green tea may lead to clinical improvement in patients with chronic lymphocytic leukemia (CLL). Findings are published online in Leukemia Research. In the small case study, the researchers report on four patients who appeared to have an improvement in the clinical state of their disease after starting over-the-counter products containing epigallocatechin gallate (EGCG), an extract of green tea. Three of the four patients met the standard criteria used to define a response treatment for clinical trials. These same investigators had previously shown that EGCG kills leukemia cells from patients with CLL in the test tube by interrupting the communication signals they need to survive. That study was published in Blood in 2004.